Why does this produce electricity? The flow of current can be understood as the flow of ions from the more reactice metal to the less reactive metal. The ions moving from one electrode to the other creates an electrical charge which is neutralised by the flow of electrons across the wire.

Before considering the reaction of two metals, consider what happens when we place a single metal electrode in an electrolyte. Some of the metal atoms in the electrolyte go into solution as ions while the remaining electrons create a negative charge on the metal. The separation of ions and electrons leads to a separation of charge. However, this build up of charge cannot continue indefinitely because as the negative charge builds up in the metal it becomes increasingly difficult for positive metal ions to go into solution. A similar build up in positive charge in the electrolyte also prevents the build up of charge. This degree of charge build up depends on the metal and represents the work required to separate electrons from the ions. This is known as the electro neutrality principle
Similarly, if a copper strip is placed in an aquaous Copper(II)Sulfate solution the copper will also lose ions. These reactions are often written as Cu | Cu+2 this is the half-cell reaction.
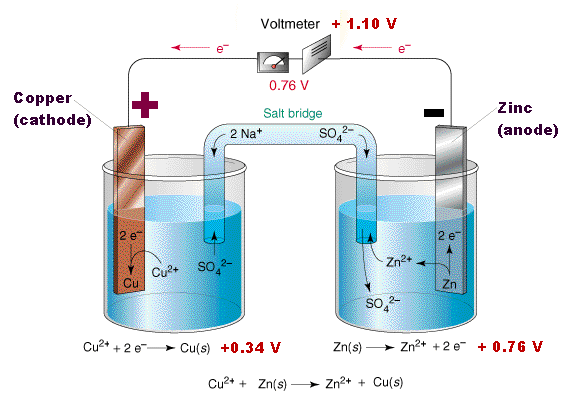
The tendancy for Zinc to lose ions is greater than that of Copper. When the two cells are joined together (using a copper wire to connect the electrodes and porous barrier that allows the ions to pass known as a salt-bridge connect the elecrolytes, the build up of electrons on the zinc will flow to through the wire onto the copper.
The copper ions in the electrolyte gain electrons and become copper atoms.
Thus the reaction can be written,
Zn | Zn2+ | | Cu2+ | Cu
No comments:
Post a Comment