Introduction
In it's most simple form a battery can be regarded as a pump that provides the energy to move charge around a circuit.
In order to provide a potential difference, or electro-motive force (EMF) a store of energy is required. One such method is a battery or cell. The common usages of the term battery is any device that converts chemical energy into electrical energy. However, strictly speaking, the term battery is used when several electrical cells are connected together to provide a source of a potential difference in a circuit. If it is just a single chemical source then it is called a cell.
History
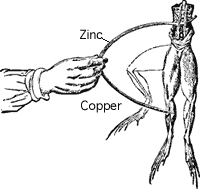
In 1791, Galvani noticed that a circuit created with two different metals, when touched on the ends of the leg of a dead frog, would cause it twitch. The two metals were creating an electric current within the frog's leg, causing the muscles to contract. Early batteries were an improvement of this method transferring chemical energy into electrical energy.
The first battery was invented in 1793 by Alessandro Volta. Just as the two different metals touching the wet skin of a frog's leg, caused an electrical current to flow, early batteries increased the voltage that could be produced by stacking a pile of discs made from silver and zinc sandwiched between paper soaked in a salt water solution as shown in Figure 2. In honour of Volta, we use the Volt as the unit of potential difference and EMF.
No comments:
Post a Comment